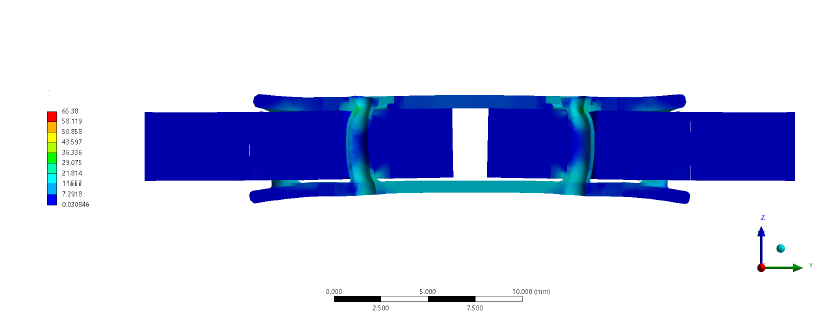
Device Breakdown
T-REMEDIE is an innovative system for tendon repair, biocompatible, biodegradable, easily implantable, minimally invasive and capable of transforming tendon tissue injuries from permanent disability to temporary discomfort, ensuring positive patient follow-ups.
Aiming to help, our purpose is to satisfy the clinical need in tendon and ligament repair by helping people to completely restore movement capability.
The System
It allows the correct restoration of the tendon tissue, ensuring a complete recovery of the patient’s range of motion. The geometry of the system facilitates its insertion, guaranteeing savings for the healthcare system in terms of costs and time. The device allows to keep the tendon stumps at a useful distance during its regeneration.
Its innovative mechanism favors a complete recovery of mobility and allows to immediately carry physiological loads. The patient can thus follow a rehabilitation protocol from the early post-surgery period. Its biocompatible and bioabsorbable material ensures both the necessary resistance during the tissue regeneration phase and the subsequent biodegradation of the material within predefined times, in accordance with the rehabilitation process.
The Technology
Biocompatible and resorbable devices for the repair of tendons and ligaments.
Functional
Ensure an adequate response under physiological loads allowing rehabilitation to begin a few days after surgery.
Sutureless & Knotless
Prevents any danger or malfunction as adhesions, slipping, knot failure and gap formation.
Biodegradable
Made of medical grade polymers of controlled degradation, that deliver safety and quality.
Easy to apply
The T-RESAP applicator perfectly coupled with the device, allows a quick, low-profile and reproducible application.
Our mission
T-REMEDIE provides a biocompatible and bioresorbable device for tendon and ligaments repair. Our technology has been designed to overcome the current-suture-based repair techniques limitations, enabling rapid and minimally invasive application in order to ensure faster recovery. This implantable medical device is made from an innovative material that combines excellent mechanical properties with a degradation time comparable to the duration of rehabilitation protocols for tendons rupture without a risk of rejection.

A research project born at the Politecnico di Torino in 2019, thanks to the collaboration with the University of Trento and ASL of Turin.
The founders are researchers from Politecnico di Torino supported by a team of surgeons and business experts.
T-REMEDIE hold a portfolio of various technology intellectual properties and trade secrets to create advanced devices for tendons and ligaments repair.
Why IT MATTERS?
Innovative devices for tendons and ligaments repair made of medical-grade materials.
BIODEGRADABLE
Specific polymers with controlled degradation
EASY TO APPLY
Thanks to the T-RESAP applicator that allows a quick, low-profile and reproducible application
PHYSIOLOGICAL LOAD TRANSMISSION
Our technology has been designed to re-create the physiological load on the tissue.
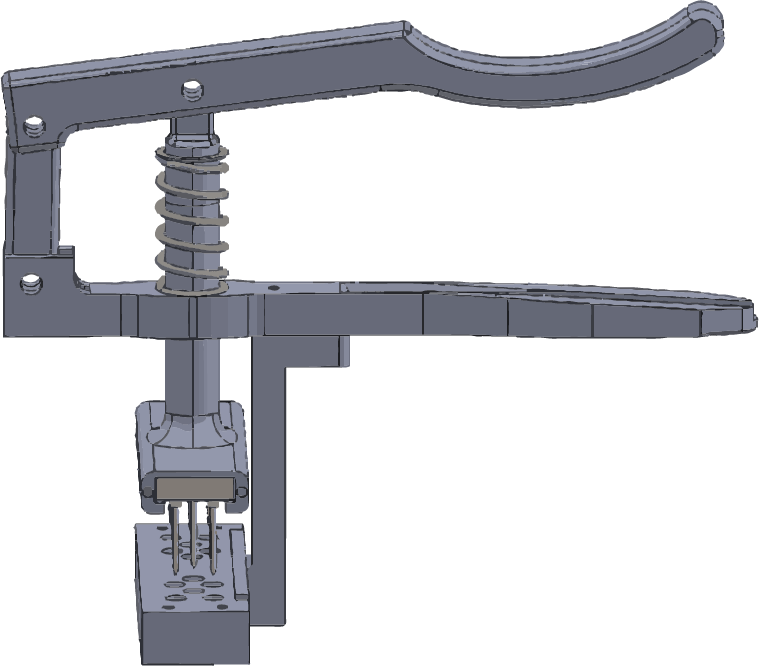
T-RESAP
This system is an instrument designed to couple with the T-REMEDIE implantable system.
It is a non-implantable instrument used to guide the drilling and insertion of the T-REM3DIE implantable device into the target tissue, facilitating automatic positioning of the entire system.
The T-RESAP applicator consists of a set of components. The device’s housing system is interchangeable so that it can be adapted to all configurations of the T-REM3DIE system depending on the size of the tendons.
Decomposable instrument in 316L stainless steel
Class I Medical Device
Reduced geometric structure and ergonomic handle
CHECK FOR SOME NEWS
THE CONTEXT
Nowadays, the limitations of current tendon and ligament repair techniques frequently result in a partial restoration of the physiological tissue’s properties and joint movement. The primary method for tendon repair is surgical suture, which employs different techniques that have been developed during the past years. The two main issues associated with the use of tendon sutures are the suture rupture itself, the development of high stresses at the interface between the suture and the tendon, and adhesions with the surrounding tissues. Other technologies available on the market regard devices that are not bioresorbable or percutaneous suture, nor biodegradable for tendon and ligament repair. Issues that arise some complications, such as a permanent reduction in the mobility of the affected joint.
In this context, the T-REMEDIE (Tendon REpair MEdical DevIcE) initiative was created in response to a clinical need identified by Dr. Federica Bergamin, an orthopaedic surgeon at Ivrea Hospital – ASLTO4. T-REMEDIE is an implantable, biodegradable medical device. Its peculiar and innovative geometry allows to overcome all the issues related with the use of current tendon repair techniques (traditional sutures and other medical devices).
Tendon injuries represent a considerable economic and social burden for both the healthcare system and patients involved. Tendon repair remains a challenge despite the important advances in surgical techniques, and protocols of post-operative rehabilitation. Hand, Achilles and Rotator cuff tendons are the most prevalent injuries worldwide. The patent allows the protection of the device configuration for Achilles and hand flexor tendons repair. According to the Hospital Discharge Register (HDR) data, for Italy alone in 2019 there were around 6000 Achilles tendon ruptures.
However, considering the medical literature the incidence rate is 23.3/100 000 injuries forr habitat. Instead, there were around 9000 open wounds cases for hand and wrist with tendon involvement in 2019 (HDR Italy). Furthermore, the Italian representative of the SINIACA-IDB (S-IDB) reported 20568 cases per year of hand flexor rupture or laceration in the Italian emergency departments. From the information collected, the addressable market for the application on Achilles tendon is estimated between 6000 and 12000 cases per year and between 9000 and 20568 cases per year for hand flexor tendon.
To commercialize a medical device in the European and American countries some certifications are required to obtain CE Mark and FDA Mark before the market entry. T-REMEDIE is a class III medical device, which is subjected to the most stringent requirement. T-RESAP system, instead, is a class I medical device, and it does not require a clinical evaluation study. Further crucial activities are related to the device manufacturing and its commercialization. In particular: the device and applicator manufacturing including packaging and final sterilization will be outsourced to a manufacturing company. The biomedical material to make the devices will be acquired from a leading industry in the market.
The market entry strategy in Europe and Worldwide will be based on the involvement of distributors of medical devices allowing the commercialization. Moreover, the T-REMEDIE adoption could be facilitated through the support of the opinion leaders who will be involved during the device’s clinical trials phase. The clinical adoption of the T-REMEDIE will increase the outcome of the surgery allowing an early return to daily-life activities. And moreover, reduces the economic burden on the National Health System and the patient.
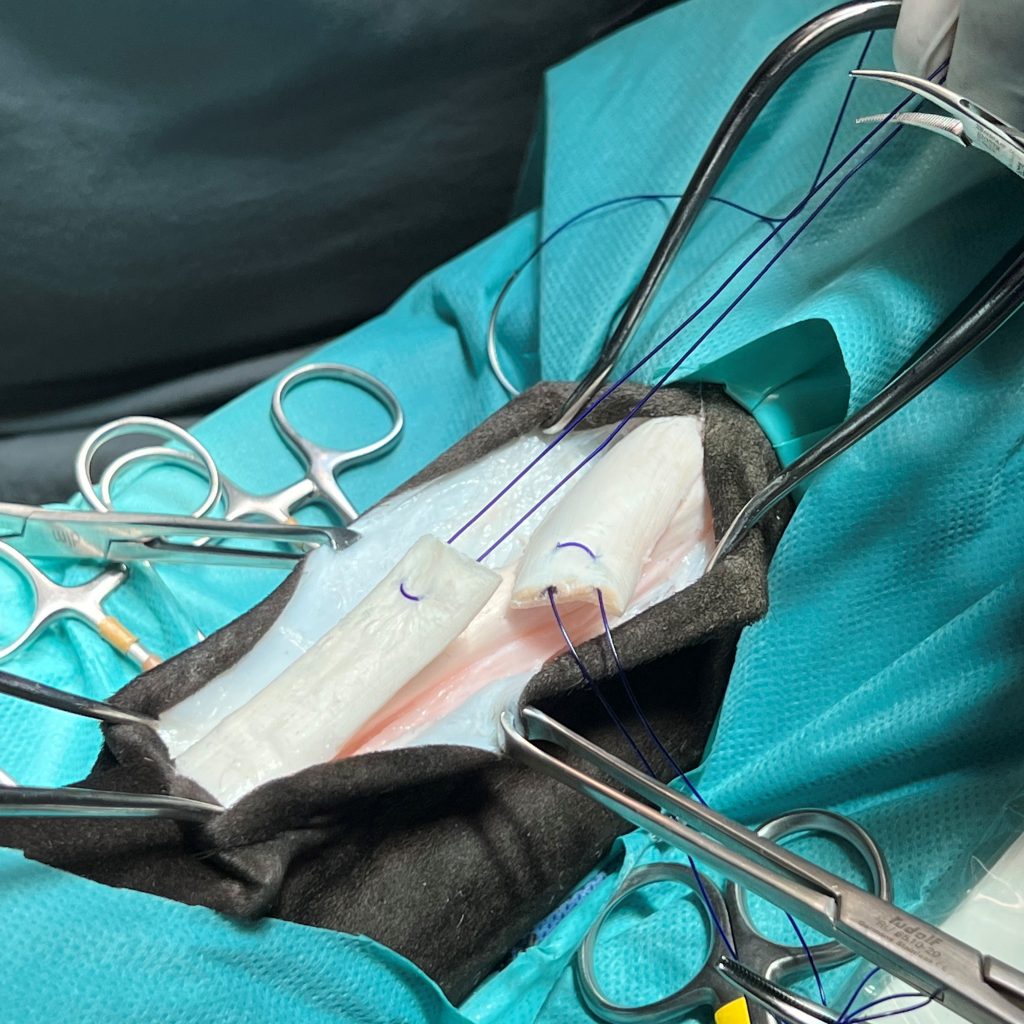
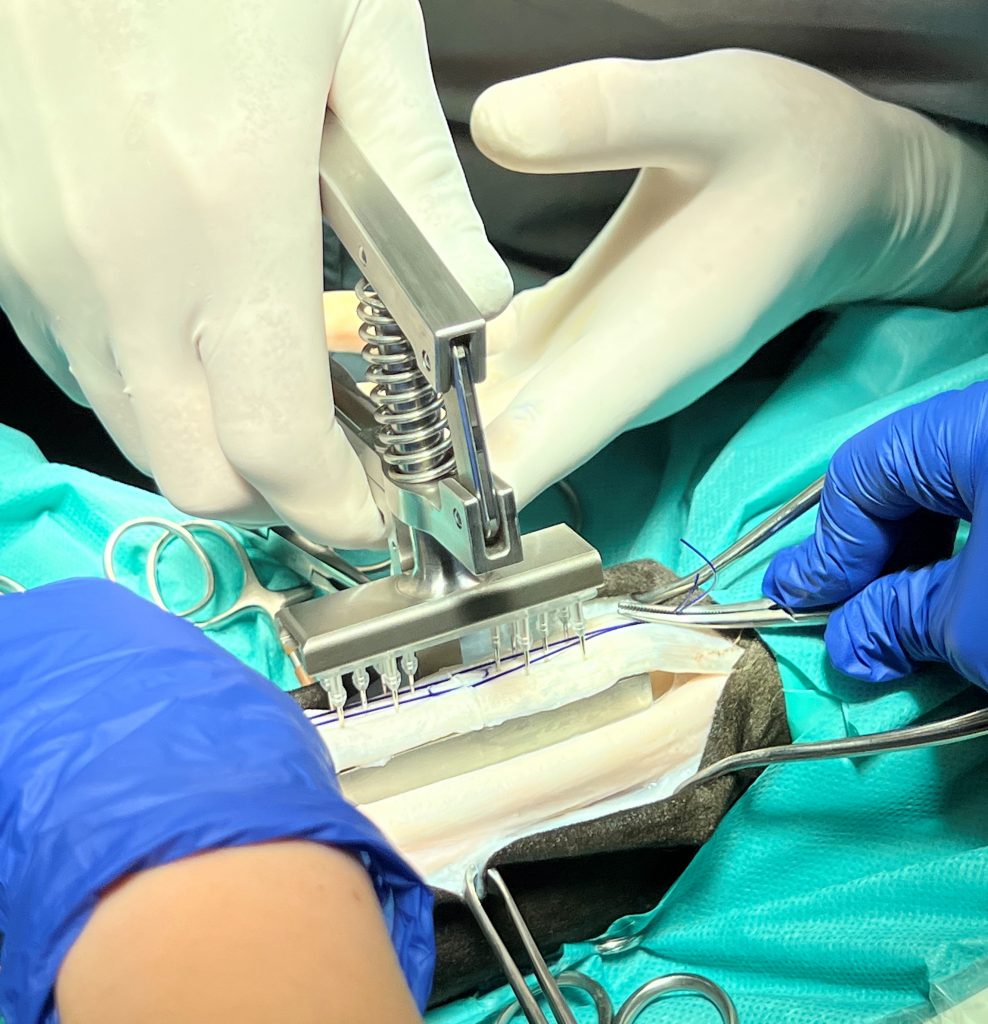
Animal Lab: ex vivo test on equin tendon
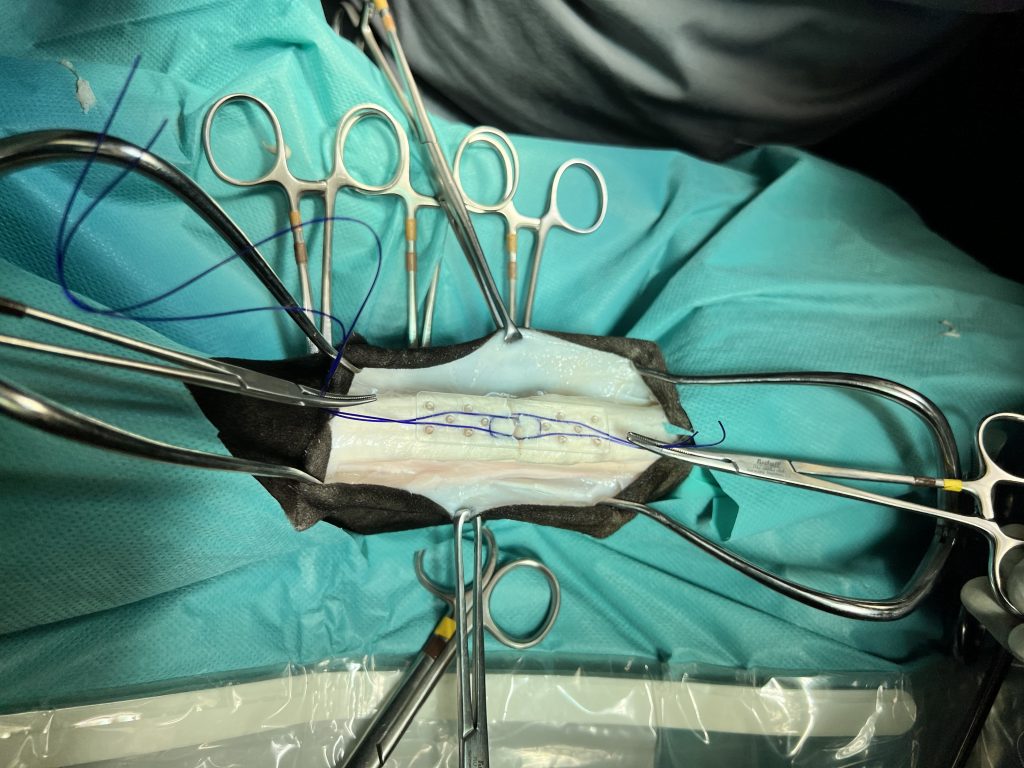
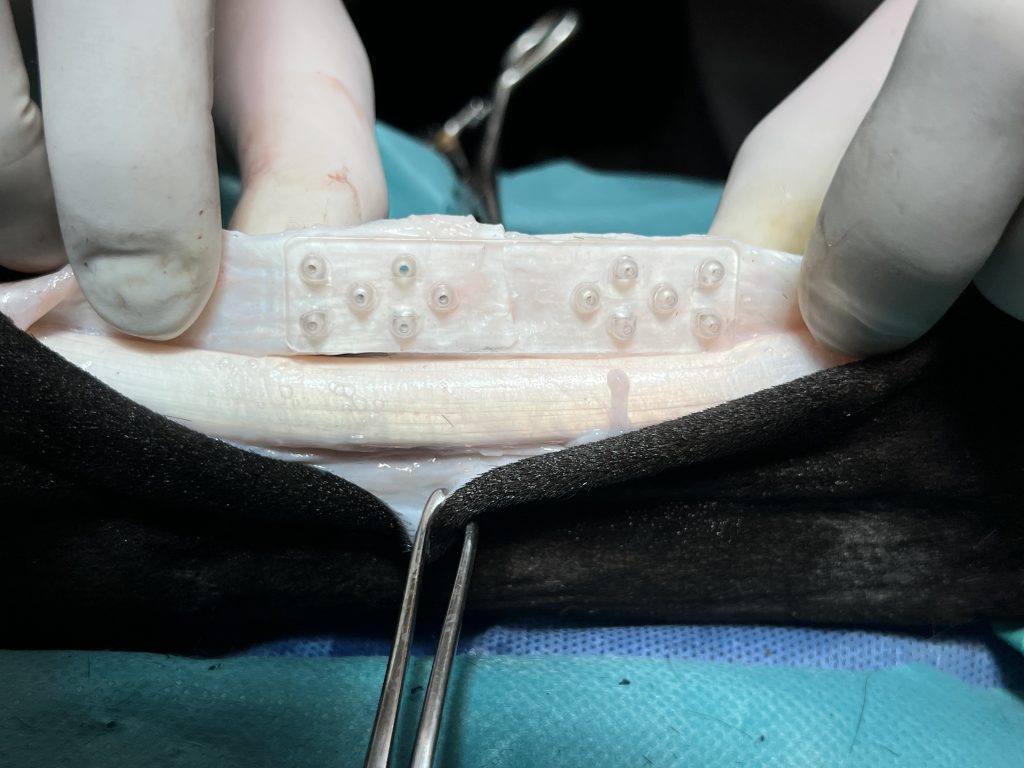
Finite element analysis: tendon and device